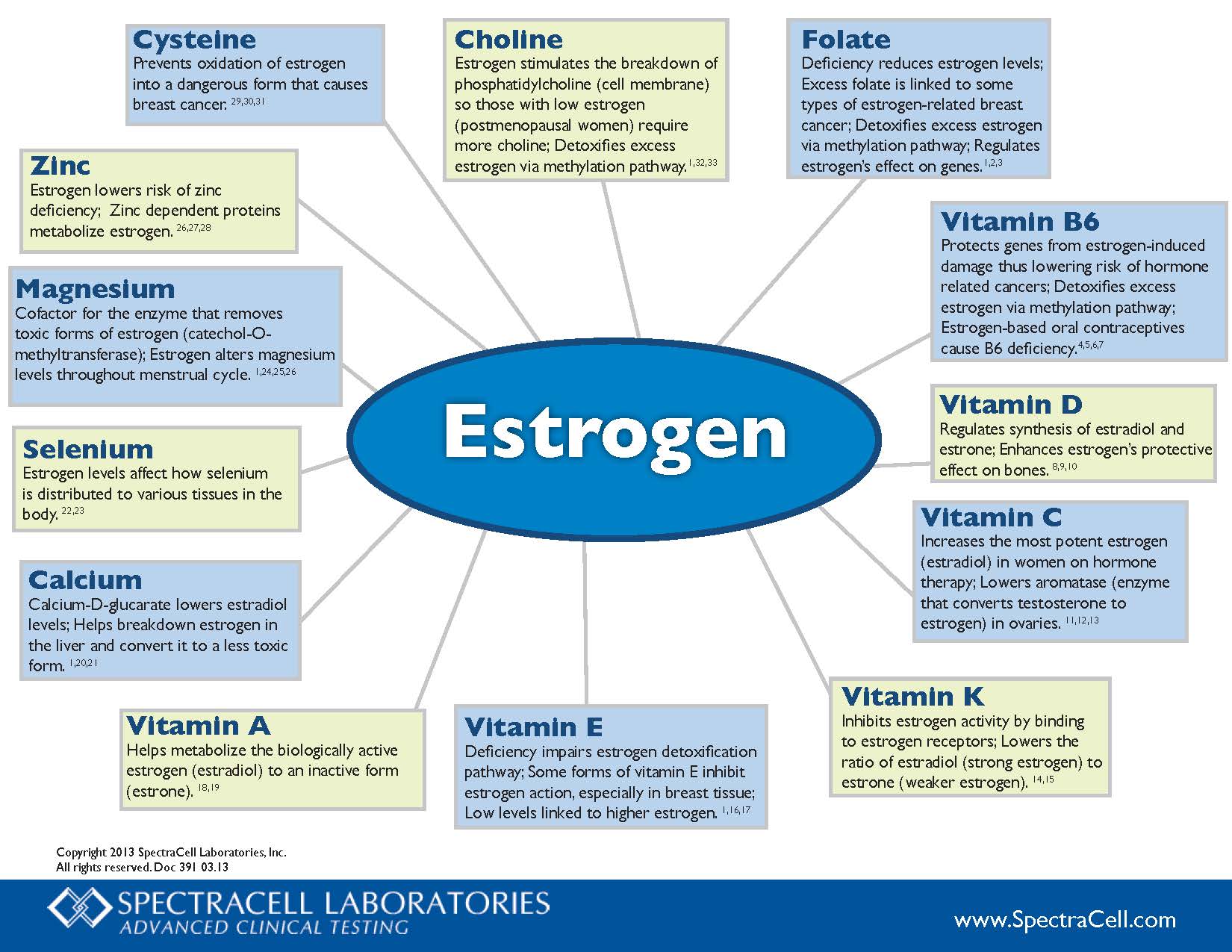
Choline – Estrogen stimulates the breakdown of phosphatidylcholine (cell membrane) so those with low estrogen (postmenopausal women) require more choline; Detoxifies excess estrogen via methylation pathway.1,32,33
Folate – Deficiency reduces estrogen levels; Excess folate is linked to some types of estrogen-related breast cancer; Detoxifies excess estrogen via methylation pathway; Regulates estrogen’s effect on genes.1,2,3
Vitamin B6 – Protects genes from estrogen-induced damage thus lowering risk of hormone related cancers; Detoxifies excess estrogen via methylation pathway; Estrogen-based oral contraceptives cause B6 deficiency.4,5,6,7
Vitamin D – Regulates synthesis of estradiol and estrone; Enhances estrogen’s protective effect on bones.8,9,10
Vitamin C – Increases the most potent estrogen (estradiol) in women on hormone therapy; Lowers aromatase (enzyme that converts testosterone to estrogen) in ovaries.11.12.13
Vitamin K – Inhibits estrogen activity by binding to estrogen receptors; Lowers the ratio of estradiol (strong estrogen) to estrone (weaker estrogen).14,15
Vitamin E – Deficiency impairs estrogen detoxification pathway; Some forms of vitamin E inhibit estrogen action, especially in breast tissue; Low levels linked to higher estrogen.1,16,17
Vitamin A – Helps metabolize the biologically active estrogen (estradiol) to an inactive form (estrone).18,19
Calcium – Calcium-D-glucarate lowers estradiol levels; Helps breakdown estrogen in the liver and convert it to a less toxic form.1,20,21
Selenium – Estrogen levels affect how selenium is distributed to various tissues in the body.22,23
Magnesium – Cofactor for the enzyme that removes toxic forms of estrogen (catechol-O-methyltransferase); Estrogen alters magnesium levels throughout menstrual cycle.1,24,25,26
Zinc – Estrogen lowers risk of zinc deficiency; Zinc dependent proteins metabolize estrogen.26,27,28
Cysteine – Prevents oxidation of estrogen into a dangerous form that causes breast cancer.29,30,31