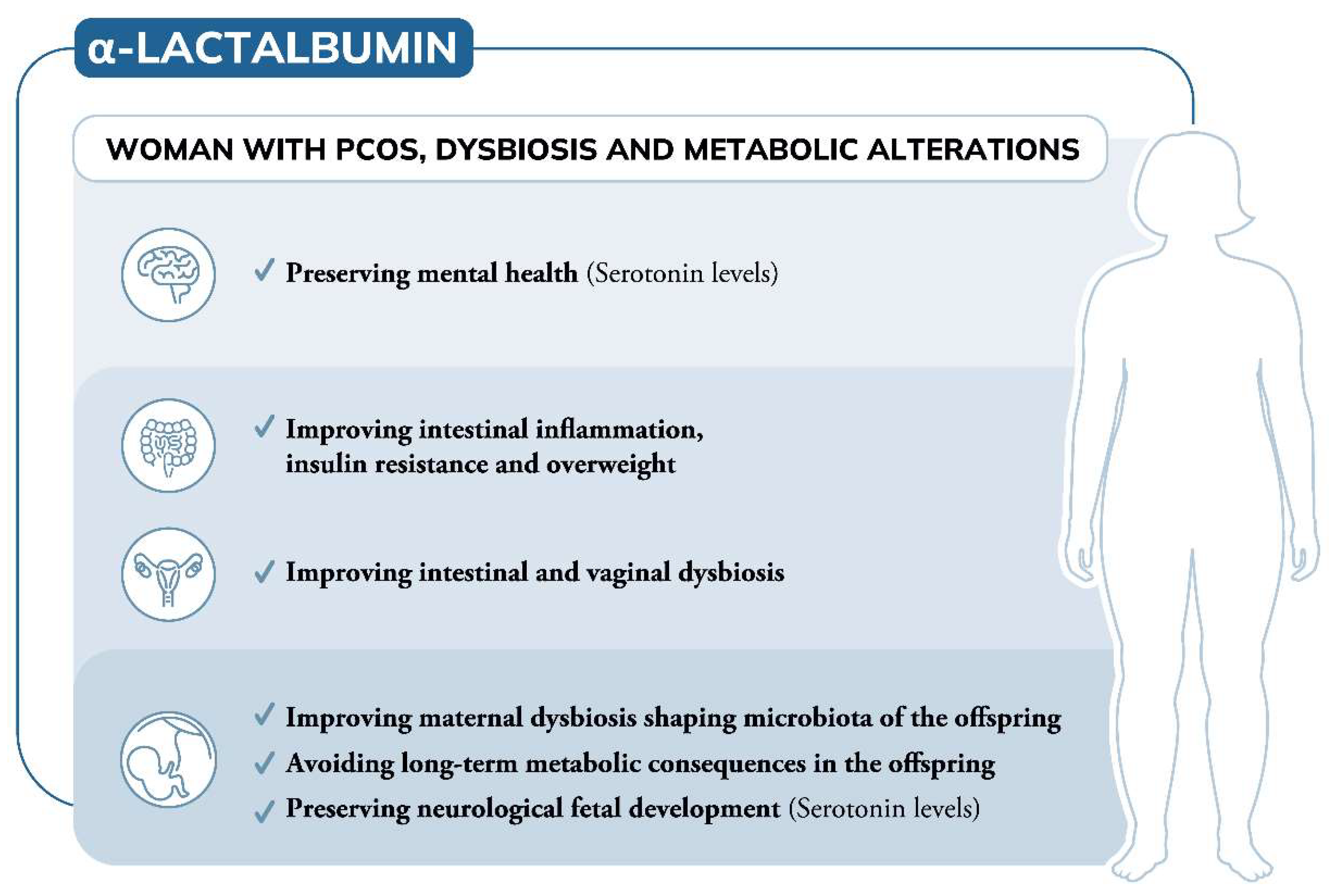
To date, the involvement of α-Lactalbumin (α-LA) in the management of polycystic ovary syndrome (PCOS) refers to its ability to improve intestinal absorption of natural molecules like inositols, overcoming the inositol resistance. However, due to its own aminoacidic building blocks, α-LA is involved in various biological processes that can open new additional applications.
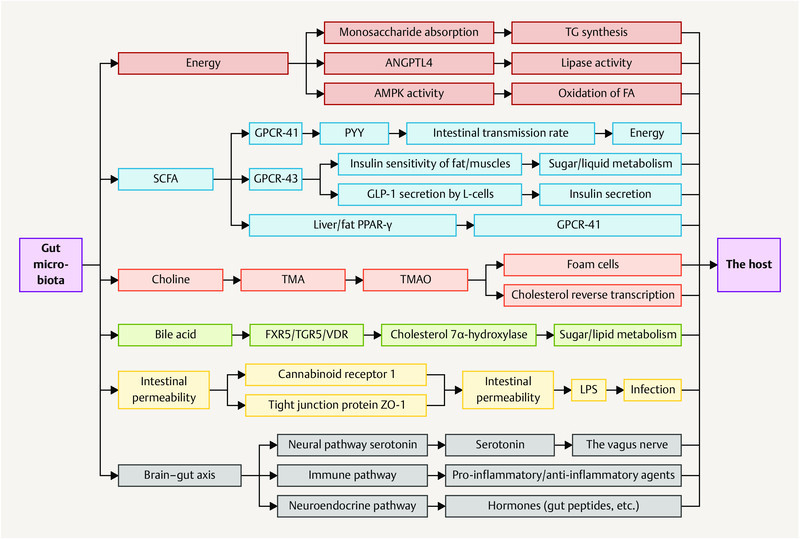
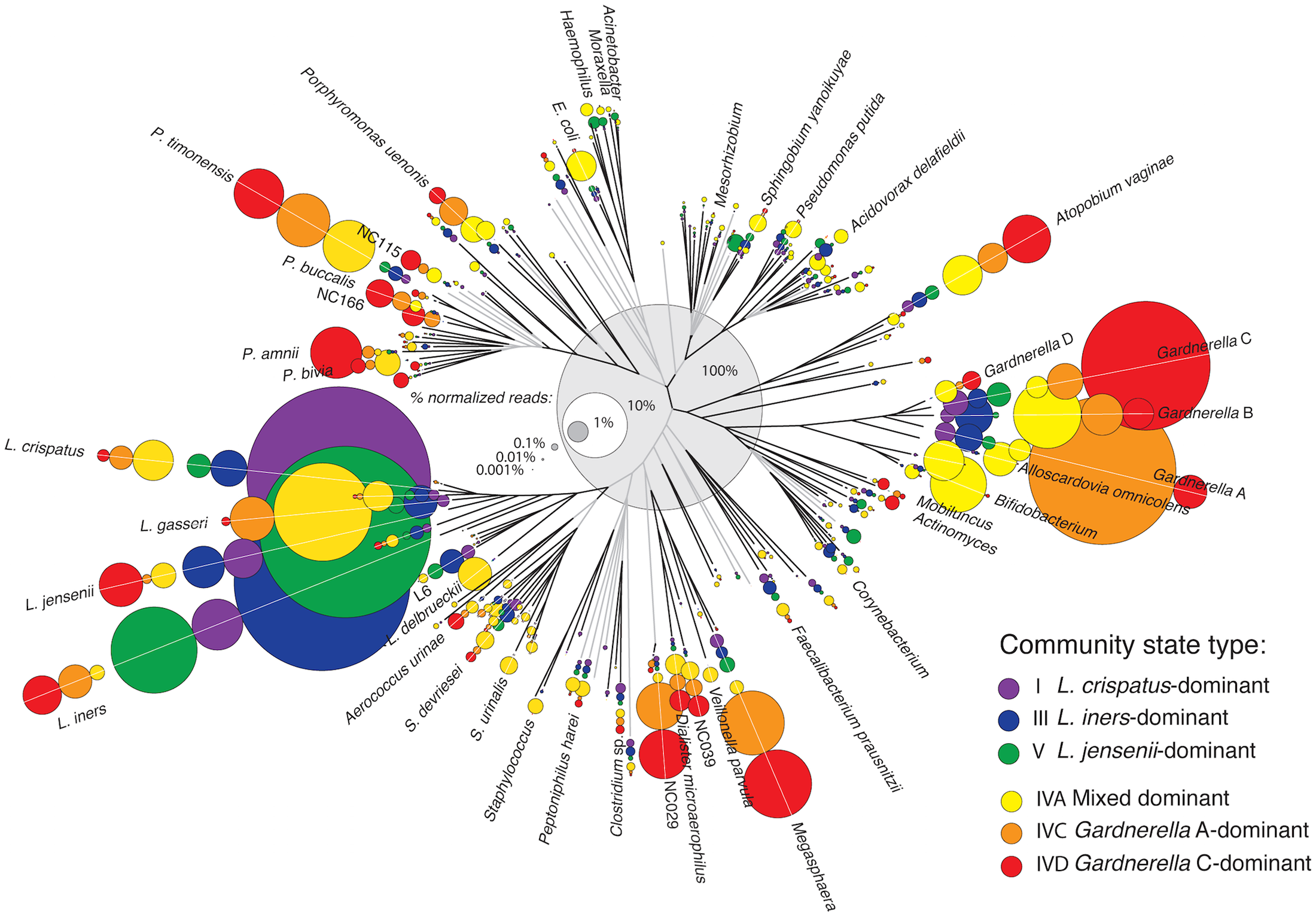
A great portion of women with PCOS exhibit gastrointestinal dysbiosis, which is in turn one of the triggering mechanisms of the syndrome. Due to its prebiotic effect, α-LA can recover dysbiosis, also improving the insulin resistance, obesity and intestinal inflammation frequently associated with PCOS. Further observations suggest that altered gut microbiota negatively influence mental wellbeing.
Depressive mood and low serotonin levels are indeed common features of women with PCOS. Thanks to its content of tryptophan, which is the precursor of serotonin, and considering the strict link between gut and brain, using α-LA contributes to preserving mental well-being by maintaining high levels of serotonin.
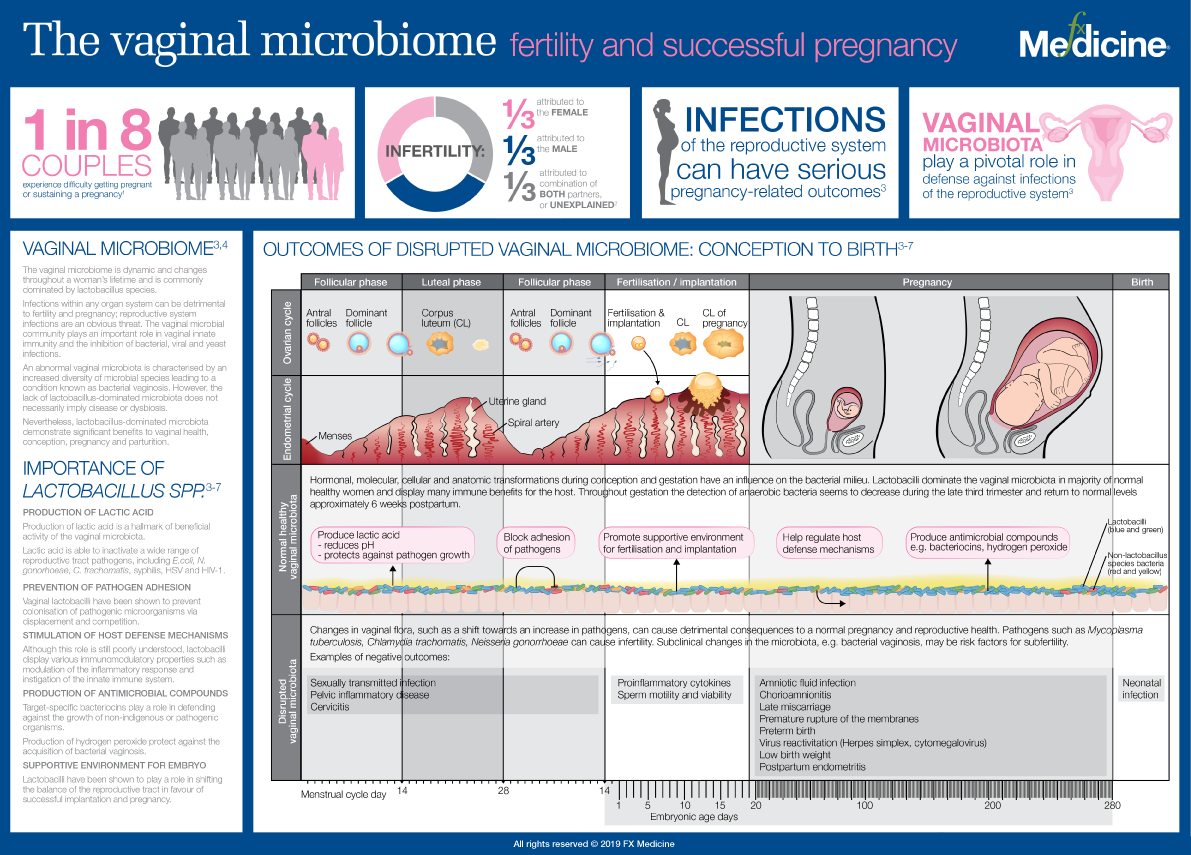
In addition, considering women with PCOS seeking pregnancy, both altered microbiota and serotonin levels can induce later consequences in the offspring. Therefore, a deeper knowledge of potential applications of α-LA is required to transition to preclinical and clinical studies extending its therapeutic advantages in PCOS.
NOTE: α-LA is high in whey protein