Highlights
- Transmission of PCOS traits in mice occurs via an altered DNA methylation landscape
- Metabolic- and inflammatory-related pathways are dysregulated in models of PCOS
- Common hypomethylation signatures occur in a mouse model of PCOS and in humans
- Identification of a novel epigenetic-based therapeutic strategy for PCOS
Summary
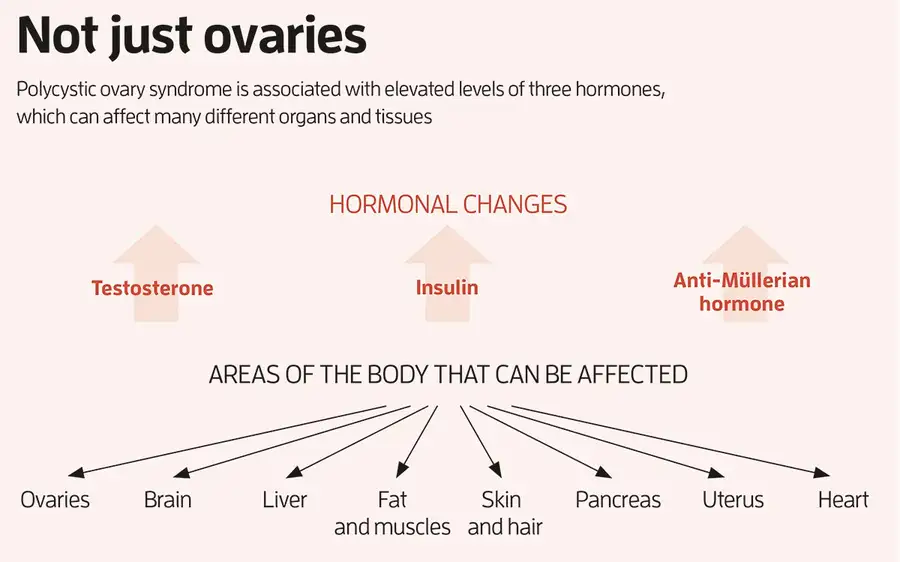
Polycystic ovary syndrome (PCOS) is the most common reproductive and metabolic disorder affecting women of reproductive age. PCOS has a strong heritable component, but its pathogenesis has been unclear. Here, we performed RNA sequencing and genome-wide DNA methylation profiling of ovarian tissue from control and third-generation PCOS-like mice.
We found that DNA hypomethylation regulates key genes associated with PCOS and that several of the differentially methylated genes are also altered in blood samples from women with PCOS compared with healthy controls. Based on this insight, we treated the PCOS mouse model with the methyl group donor S-adenosylmethionine and found that it corrected their transcriptomic, neuroendocrine, and metabolic defects.
These findings show that the transmission of PCOS traits to future generations occurs via an altered landscape of DNA methylation and propose methylome markers as a possible diagnostic landmark for the condition, while also identifying potential candidates for epigenetic-based therapy.
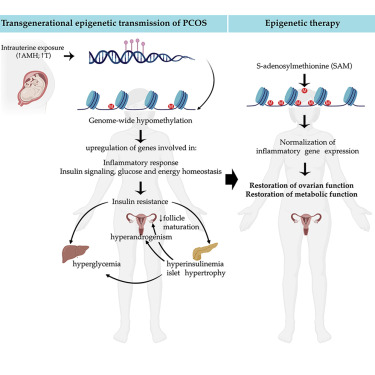
Discussion
We speculate that a global loss of DNA methylation, particularly in promoter-TSS and upstream-promoters, could be responsible for genomic instability in the disease condition. Consistently, a genome-wide DNA methylation study on umbilical cord blood reports a prevalence of hypomethylation in women with PCOS compared with unaffected women (Lambertini et al., 2017). As genomic instability is highly correlated with DNA damage, excessive DNA demethylation could be thus associated with impaired DNA damage repair. This is in line with many reports describing a strong association between PCOS and malignancies, such as ovarian and endometrial cancer (Escobar-Morreale, 2018), and suggest that a higher predisposition to cancer detected in women with PCOS could be due to altered DNA methylation landscapes.
Remarkably, we report that several of the differentially methylated genes identified in ovarian tissues of PCOS mice of the third generation are also altered in blood samples from women with PCOS and from daughters of women with PCOS compared with healthy women. Six genes associated with DNA demethylation (TET1), axon guidance (ROBO-1), inhibition of cell proliferation (CDKN1A), inflammation (HDC), and insulin signaling (IGFBPL1, IRS4) are hypomethylated in women with PCOS as compared with controls, and three genes (ROBO-1, HDC, and IGFBPL1) are also hypomethylated in daughters diagnosed with PCOS.
Here, we examined the therapeutic potential of SAM, a known natural agent causing methylation of several genes (Chik et al., 2014). To our knowledge, this is the first direct evidence for the potential therapeutic effect of SAM in a preclinical model of PCOS. Our investigation showed that SAM treatment can rescue the major PCOS reproductive neuroendocrine and metabolic alterations of PAMH F3 mice, thus highlighting the therapeutic potential of methylating agents as promising epigenetic therapies aimed at treating women with PCOS. We provide evidence that the methylating agent restores the aberrant expression of most inflammatory genes investigated in the ovaries as well as in metabolic tissues of PAMH F3 adult mice. Numerous studies show a causal link between low-grade inflammation and metabolic diseases, including T2D (Reilly and Saltiel, 2017). Moreover, the degree of inflammation correlates well with the severity of insulin resistance, T2D, and hyperandrogenism related to PCOS (González et al., 2006; Zhao et al., 2015).
Based on our findings we can speculate that the trigger for tissue inflammations could emanate from altered DNA methylation landscapes, which can be corrected by the SAM.
Taken together, this study points to AMH excess during gestation as a detrimental factor leading to the transgenerational transmission of PCOS cardinal neuroendocrine, reproductive, and metabolic alterations and shed lights into the epigenetic modifications underlying the susceptibility of the disease while pointing to novel diagnostic tools and epigenetic-based therapeutic avenues to treat the disease.