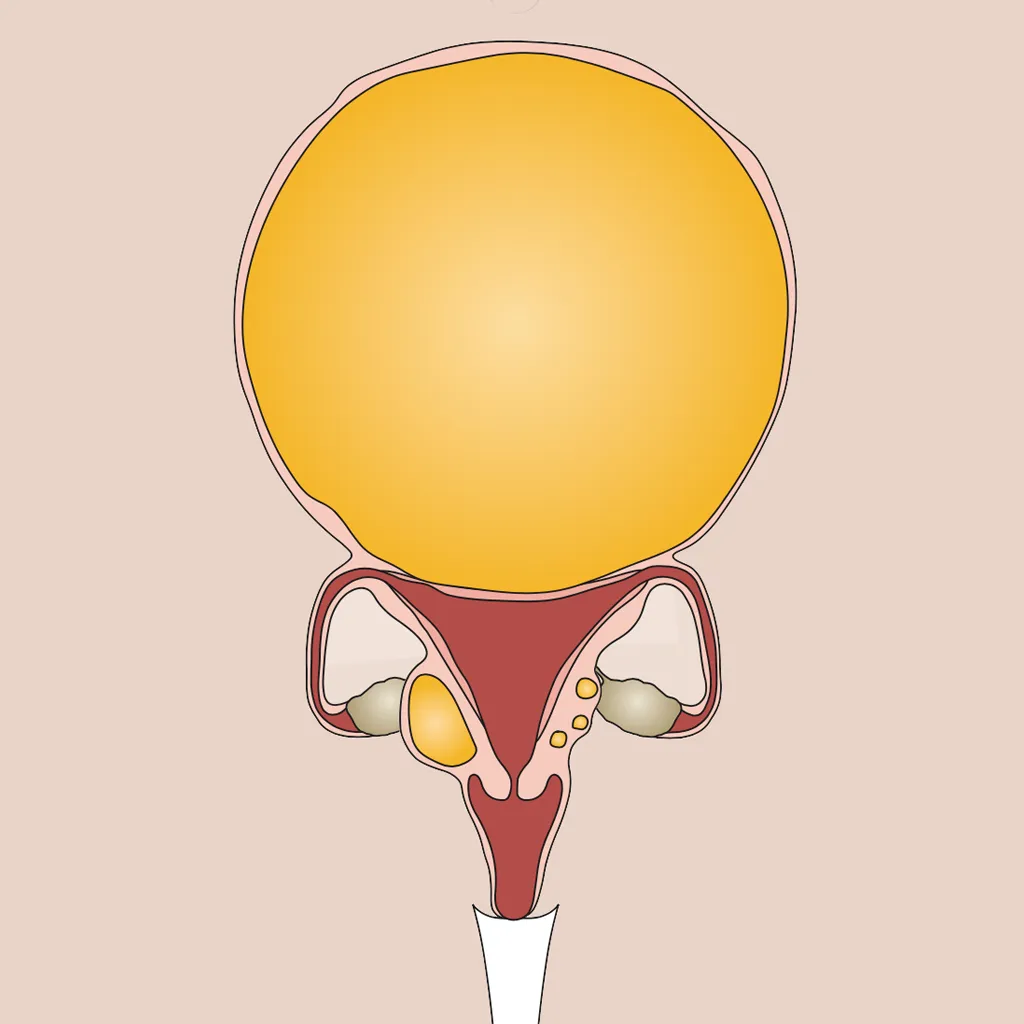
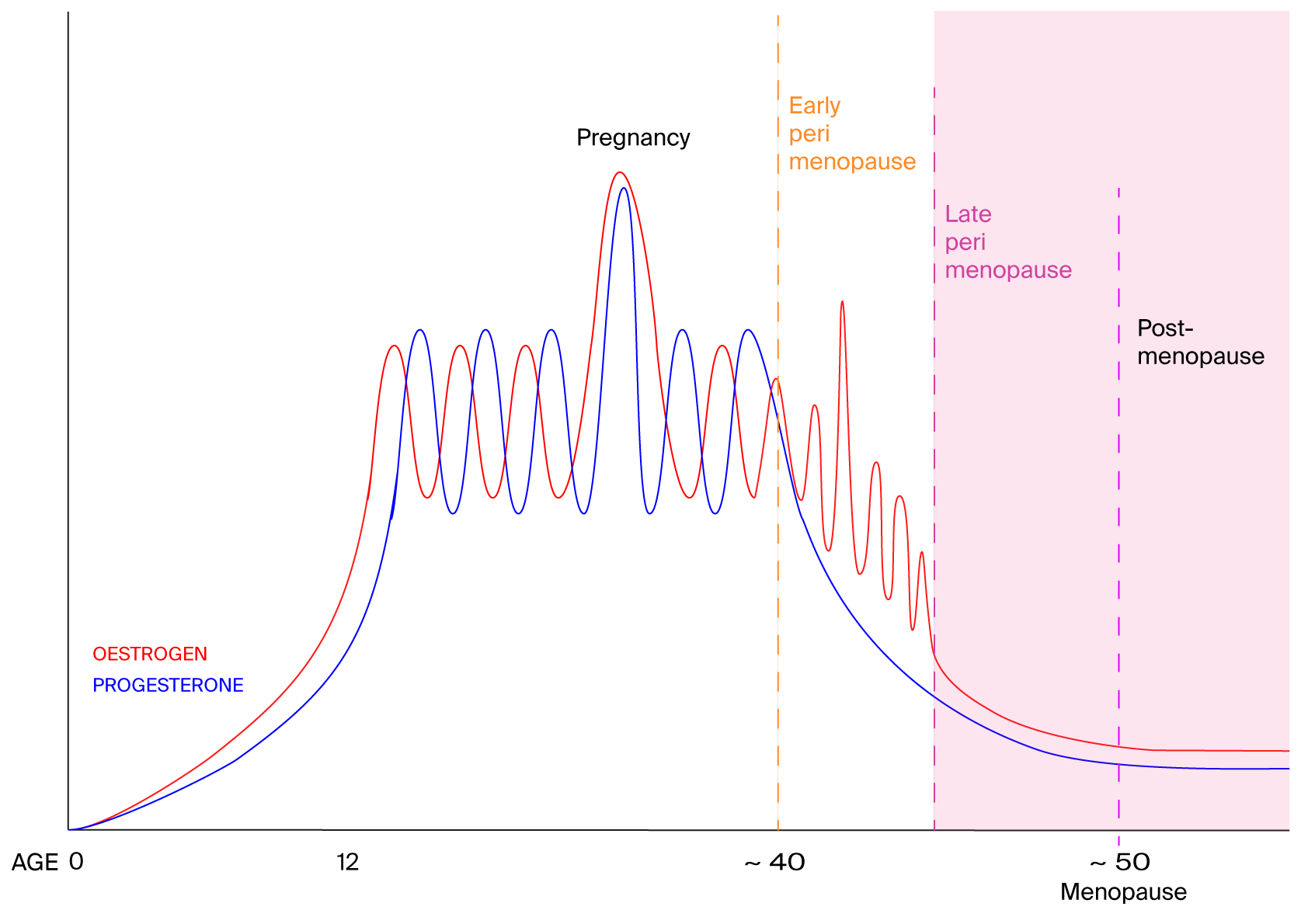
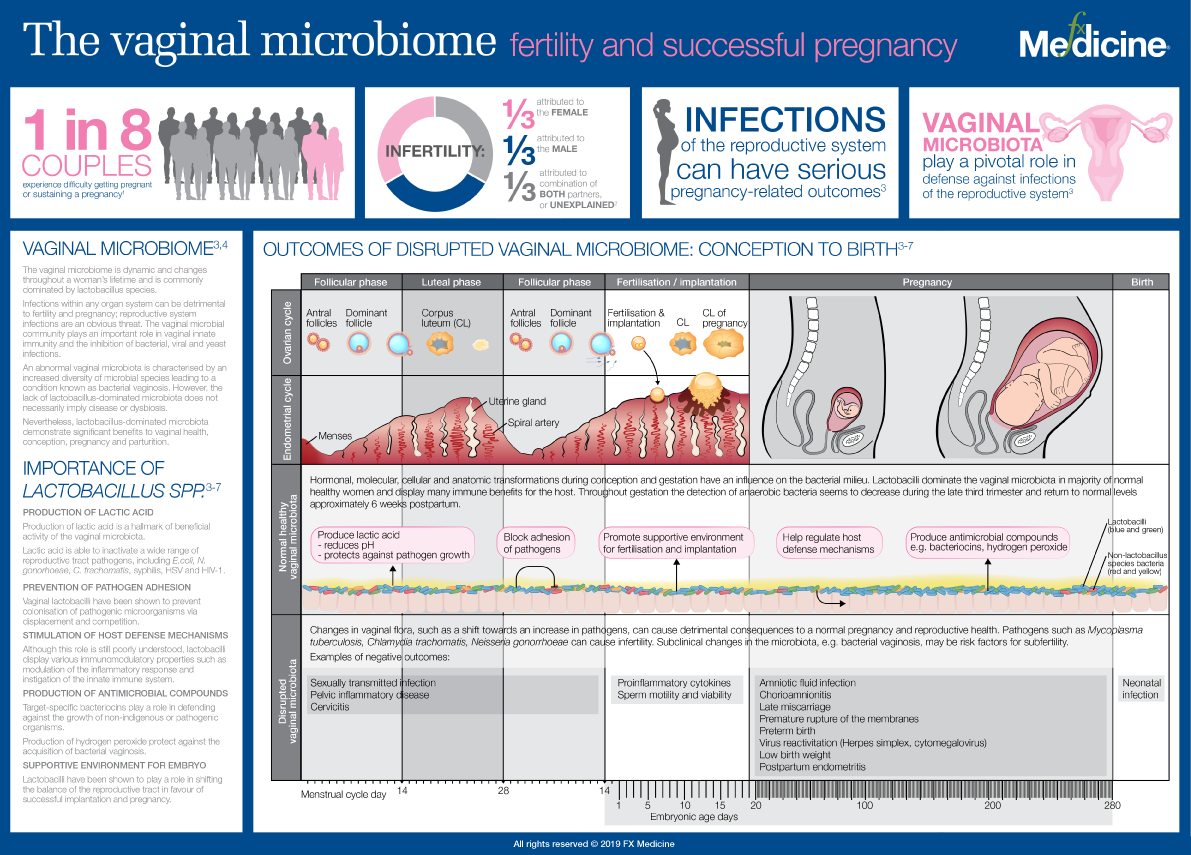
Reduced levels of oestrogen and progesterone seem to be what makes post-menopausal women more likely to have symptoms of sleep apnoea, including snoring, irregular breathing or gasping at night.
Middle-aged women who have lower levels of oestrogen and progesterone are more likely to snore, breathe irregularly and gasp while sleeping, which are all symptoms of sleep apnoea.
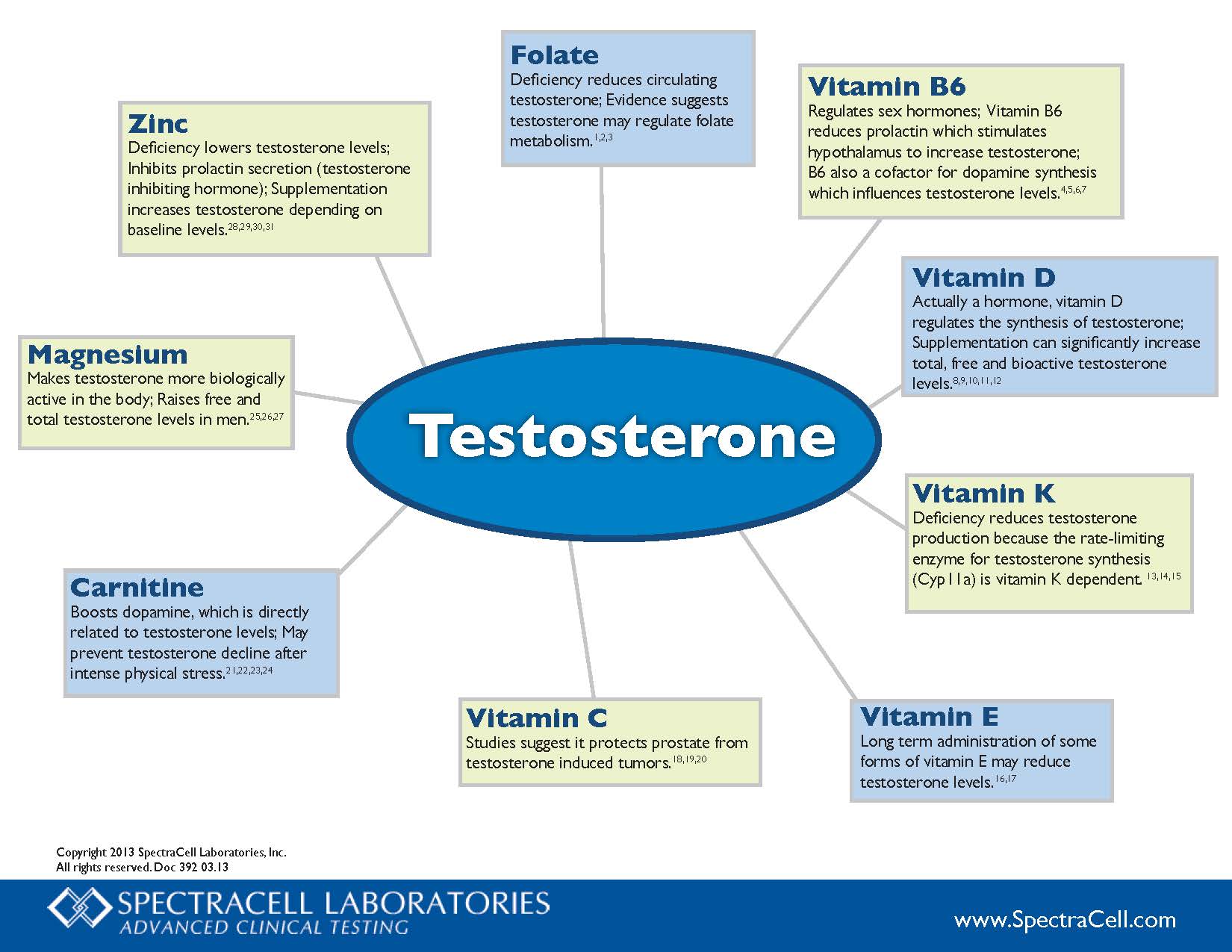
The involvement of these chemicals means targeted hormone therapy might prove useful for post-menopausal women, says Kai Triebner at the University of Bergen in Norway.
“Women live, on average, longer than men, but during later years, the quality of women’s life is comparatively low, which is inherently associated with their [low-oestrogen] hormone profile,” says Triebner. “Snoring and sleep-related breathing problems add to the burden.”
His team interviewed 774 women aged between 40 and 67 years old, mostly white, living in seven European countries about their respiratory health and lifestyles. The team also carried out clinical exams and took blood samples. The women, some of whom hadn’t yet reached menopause, completed questionnaires about their sleep habits and health. The study didn’t include pre or post-menopausal trans men.
Nearly half the women reported that they had a “disturbing snore”, says Triebner. In addition, 14 per cent had irregular breathing and 13 per cent gasped while sleeping.
Blood analyses revealed that the participants’ oestrogen and progesterone levels varied widely, ranging from just a few units per litre in some women to tens of thousands of units per litre in others. Those variations had clear associations with sleep apnoea, he says. As the levels of oestrone – a kind of oestrogen – doubled, women were 19 per cent less likely to snore. And as progesterone levels doubled, women were 9 per cent less likely to snore.
Within the group of women who snored, there was a 20 per cent drop in the chances of having irregular breathing as oestrogen levels doubled. And a doubling in progesterone levels was linked to a 12 per cent lower likelihood of waking up feeling like they are choking.
PLoS One DOI: 10.1371/journal.pone.0269569